Hepatitis C Treatment Regimen Selector
Select Your Patient's Details
Select patient details above to see treatment recommendation
Treatment Details
Treatment details will appear here based on your selection
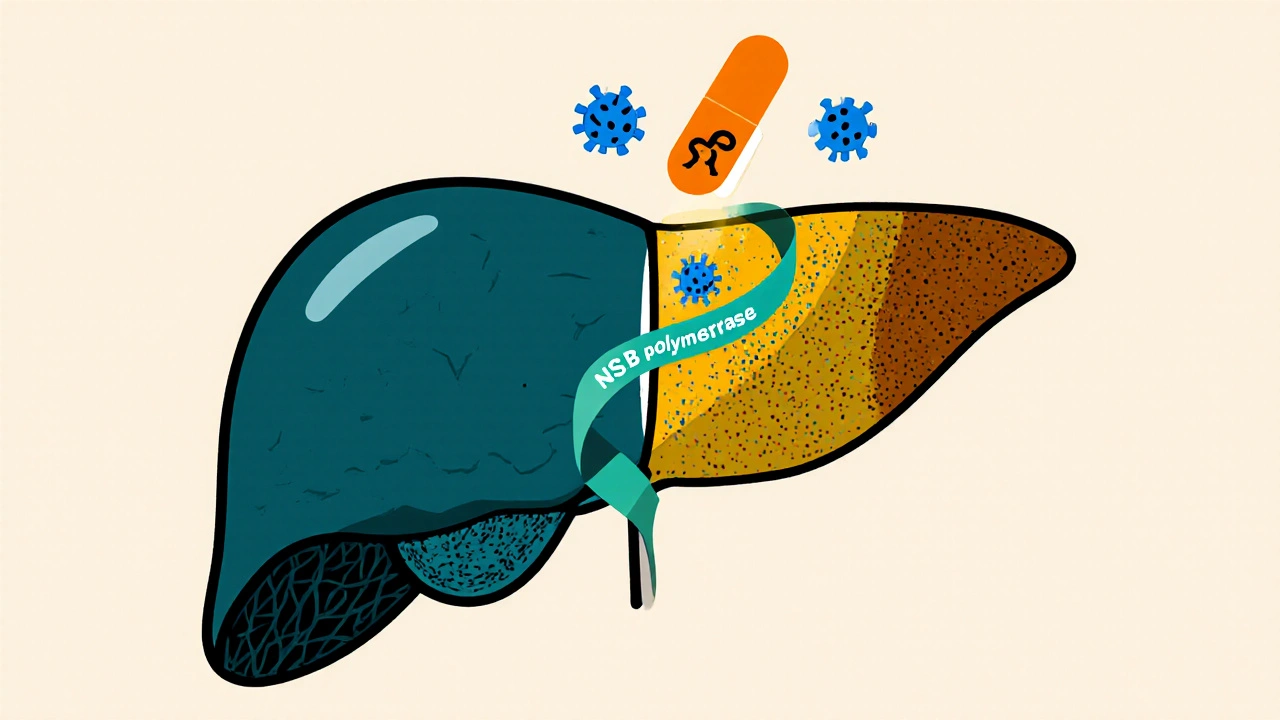
Sofosbuvir is a nucleotide‑analog inhibitor of the hepatitis C virus NS5B RNA‑dependent RNA polymerase. When paired with other direct‑acting antivirals (DAAs), it has reshaped how clinicians tackle hepatitis C and its downstream problems. Hepatitis C is a blood‑borne viral infection that primarily targets the liver and can progress to chronic disease, fibrosis, and cirrhosis. The virus exists in several genotypes, each responding slightly differently to therapy. Liver cirrhosis is the end‑stage scarring of liver tissue, often the final complication of long‑standing hepatitis C infection. Understanding how sofosbuvir fits into the broader treatment landscape helps doctors prevent, mitigate, or even reverse these complications.
Why Sofosbuvir Became a Game‑Changer
Before 2013, interferon‑based regimens dominated hepatitis C care. Those protocols required weekly injections, caused flu‑like symptoms, and achieved cure rates (SVR - sustained virologic response) of 40‑50 % for hard‑to‑treat genotypes. Sofosbuvir arrived as the first oral DAA with a high barrier to resistance, allowing once‑daily dosing and cure rates above 95 % across most genotypes.
- Pan‑genotypic activity: effective against genotypes 1‑6.
- Minimal drug‑drug interactions compared with protease inhibitors.
- Short treatment courses: 8‑12 weeks for most patients.
- Well‑tolerated: fewer side‑effects than interferon or ribavirin.
These advantages translate into real‑world benefits-fewer clinic visits, lower monitoring costs, and, most importantly, a dramatic drop in hepatitis C‑related complications.
Key Complications of Chronic Hepatitis C
Even with a virus that often feels invisible, the liver pays the price. The most common sequelae include:
- Progressive fibrosis leading to cirrhosis. \n
- Hepatocellular carcinoma (HCC) risk, which rises sharply once cirrhosis is established.
- Decompensated liver disease: ascites, hepatic encephalopathy, and variceal bleeding.
- Extra‑hepatic manifestations such as mixed cryoglobulinemia, insulin resistance, and renal impairment.
Effective antiviral therapy can halt or even reverse many of these pathways. The timing of treatment-whether before or after cirrhosis develops-greatly influences outcomes.
How Sofosbuvir‑Based Regimens Address Complications
Three therapeutic pillars define modern hepatitis C care with sofobuvir:
- Viral eradication. Achieving SVR stops ongoing liver injury, allowing scar tissue to remodel.
- Reduced inflammation. By suppressing viral replication, the inflammatory cascade that drives fibrosis diminishes.
- Lowered oncogenic pressure. Cure reduces HCC incidence by up to 80 % in patients without advanced cirrhosis.
Clinical trials (e.g., ASTRAL‑1, POLARIS‑4) documented SVR12 rates of 97‑99 % with sofobuvir + ledipasvir or sofobuvir + velpatasvir, regardless of baseline fibrosis stage. Real‑world registries in Australia and Europe echo these numbers, showing a steep decline in decompensation events within two years of cure.

Choosing the Right Sofosbuvir Combination
While sofobuvir can stand alone, guidelines from the World Health Organization recommend pairing it with a second DAA tailored to genotype and liver status. Below is a quick decision matrix:
| Genotype | Compensated Cirrhosis? | Recommended Regimen | Treatment Duration |
|---|---|---|---|
| 1, 2, 3, 4, 5, 6 | No | Sofosbuvir + Velpatasvir | 12 weeks |
| 1 | Yes | Sofosbuvir + Velpatasvir | 12 weeks (extend to 24 weeks if prior treatment failure) |
| 3 | Yes | Sofosbuvir + Glecaprevir/Pibrentasvir | 12 weeks |
| Any | Decompensated | Consult hepatology; often Sofosbuvir + Ribavirin (if tolerated) or liver transplant evaluation | Varies |
Note that ribavirin, though older, still plays a role in specific rescue scenarios. Its side‑effects-anemia and teratogenicity-require close monitoring.
Monitoring and Managing Side‑Effects
Even the most tolerable drugs can cause issues. Common adverse events with sofobuvir combos include headache, fatigue, and mild nausea. Rarely, patients experience elevated liver enzymes, especially if baseline ALT is already high.
- Baseline labs: HCV RNA, genotype, liver function tests (ALT, AST, bilirubin), platelet count, and APRI/FIB‑4 scores.
- During therapy: Check labs at week 4 and week 8 for cirrhotic patients; otherwise, routine monitoring is optional.
- Post‑treatment: SVR confirmation at 12 weeks after the last dose; repeat imaging (ultrasound or MRI) for HCC surveillance if cirrhosis was present.
If anemia develops while on ribavirin, dose reduction or erythropoietin support may be needed. For patients with renal impairment, dose adjustments are unnecessary for sofobuvir itself, but concomitant agents must be reviewed.
Impact on Long‑Term Outcomes
Meta‑analyses published up to 2024 reveal that cured patients experience:
- 15‑20 % reduction in all‑cause mortality compared with untreated peers.
- >70 % drop in HCC incidence for those treated before cirrhosis.
- Improved quality‑of‑life scores (SF‑36) within six months of cure.
For those already cirrhotic, SVR still lowers the risk of decompensation by roughly 50 %, highlighting the value of treating even advanced disease.
Practical Checklist for Clinicians
- Confirm HCV genotype and fibrosis stage.
- Select the appropriate sofobuvir‑based regimen per WHO guideline matrix.
- Screen for contraindications: pregnancy, severe anemia, uncontrolled cardiac disease.
- Obtain baseline labs and calculate APRI/FIB‑4.
- Educate patient on adherence (99 %+ adherence needed for cure).
- Schedule interim labs at week 4 (if cirrhotic) and final SVR test at week 12 post‑treatment.
- Continue HCC surveillance for any patient with pre‑existing cirrhosis, regardless of SVR.
Frequently Asked Questions
Can Sofosbuvir cure hepatitis C in all genotypes?
Yes. When paired with a pan‑genotypic partner like velpatasvir, sofobuvir achieves >95 % SVR across genotypes 1‑6, even in patients with cirrhosis.
Is treatment still worthwhile for patients with decompensated cirrhosis?
While cure rates slightly drop, achieving SVR can stabilize liver function and make patients eligible for transplant. Individual assessment is essential.
What are the most common side‑effects?
Headache, fatigue, and mild nausea occur in <10 % of patients. When ribavirin is added, anemia becomes a notable concern.
Do I need to continue any medication after cure?
Antiviral therapy stops after the prescribed course. However, patients with prior cirrhosis must keep up regular imaging for HCC surveillance.
How does Sofosbuvir compare to older interferon regimens?
Interferon required weekly injections, caused flu‑like symptoms, and had cure rates around 50 %. Sofosbuvir is oral, better tolerated, and lifts cure rates above 95 %.
Bottom Line
Sofosbuvir has turned hepatitis C from a chronic, life‑threatening disease into a curable condition for the vast majority of patients. By eradicating the virus early, clinicians can prevent fibrosis, lower liver‑cancer risk, and improve long‑term survival. Even for those with established cirrhosis, the drug offers a realistic chance to halt progression and regain quality of life. The key is timely diagnosis, genotype‑aware regimen selection, and diligent follow‑up after cure.

Comments
Christopher Burczyk
When assessing the clinical impact of sofosbuvir, one must first note its status as a nucleoside‑analog inhibitor that targets the NS5B polymerase with a high genetic barrier to resistance. The once‑daily oral formulation eliminates the logistical burdens associated with interferon‑based regimens, thereby improving adherence rates across diverse patient populations. Pharmacodynamic data demonstrate pan‑genotypic efficacy, rendering it suitable for genotypes 1 through 6 without the need for genotype‑specific dosing adjustments. Moreover, the minimal cytochrome P450 interaction profile permits concomitant use of many cardiovascular and antidiabetic agents, a consideration often overlooked in community practice. Consequently, the drug not only elevates sustained virologic response (SVR) percentages but also attenuates the progression of fibrosis, which translates into measurable reductions in cirrhosis‑related morbidity.
October 19, 2025 AT 20:53
Nicole Boyle
Yeah, the data pretty much scream “no more interferon nightmares,” especially when you throw in real‑world registry outcomes from Europe and Australia. The simplification of monitoring-just a baseline panel and a week‑4 check for cirrhotics-means clinics can shift resources toward proactive HCC surveillance rather than chasing side‑effects. In practice, I’ve seen patients who were previously “non‑candidates” for treatment finally get on a 12‑week sofosbuvir‑based course and bounce back with near‑normal liver enzymes. That’s the kind of paradigm shift that turns a historically fatal disease into a manageable chronic condition.
October 20, 2025 AT 03:50
Caroline Keller
Honestly this cure hype feels like a cheat code for health‑obsessed fanatics.
October 20, 2025 AT 10:46
dennis turcios
While the enthusiasm is understandable, it is prudent to acknowledge that the registry data still suffer from selection bias, as patients enrolled in those programs often have better baseline socioeconomic support. The assertion that monitoring can be “just a baseline panel” overlooks the subtleties of managing portal hypertension in decompensated cirrhosis, where more frequent imaging may be warranted. Therefore, a balanced interpretation of the evidence should temper the exuberant narrative.
October 20, 2025 AT 17:43
Felix Chan
Super exciting stuff! Seeing so many people finally get a shot at a cure makes the whole field feel like it’s finally catching up with the science. Keep the good vibes rolling, and let’s spread the word-early treatment really does save lives.
October 21, 2025 AT 00:40
Thokchom Imosana
One has to wonder, beneath the glossy press releases, what the pharmaceutical consortium is really pulling off with sofosbuvir. The drug’s patent architecture is deliberately convoluted, ensuring that generic competitors remain shackled for years while the profit margins swell beyond what any public health ethicist would condone. Moreover, the clinical trials, though lauded for their high SVR rates, were predominantly funded by the same corporations that stand to gain from the market exclusivity. This creates an inherent conflict of interest that is seldom disclosed in peer‑reviewed articles. Add to this the quiet lobbying efforts that have shaped WHO guidelines to favor pan‑genotypic regimens, effectively sidelining cheaper, albeit less fashionable, alternatives. The result is a global healthcare ecosystem that hinges on a single molecular entity, exposing patients to supply chain vulnerabilities that are amplified during geopolitical crises. In regions where the drug is priced at several tens of thousands of dollars per course, access becomes a matter of privilege rather than a universal right. The narrative of “miraculous cure” conveniently obscures the reality that many low‑income countries rely on donor programs, which are themselves subject to shifting political winds. When donations are withdrawn, whole cohorts of patients are left without a viable therapeutic option, forcing clinicians to revert to outdated interferon‑based protocols with far‑worse tolerability. Even the reported reduction in hepatocellular carcinoma incidence must be viewed with skepticism, given that the surveillance imaging required to detect early lesions is not uniformly available, potentially skewing the statistics. Furthermore, the long‑term hepatic safety profile of sustained viral eradication remains incompletely characterized; there are emerging reports of unexpected metabolic derangements post‑cure that deserve rigorous investigation. Ultimately, the promise of sofosbuvir cannot be disentangled from the broader machinations of an industry that thrives on creating dependencies rather than fostering true health equity. This is why a critical, independent appraisal of the data is essential before we declare victory.
October 21, 2025 AT 14:33
Latasha Becker
While the concerns raised are not without merit, the pharmacological data on sofosbuvir indicate a low intrinsic hepatotoxicity and a well‑characterized safety profile across Phase III trials. The meta‑analysis you referenced indeed shows a statistically significant reduction in HCC incidence among patients achieving SVR, even after adjusting for baseline fibrosis. Moreover, post‑marketing surveillance in multiple jurisdictions has not identified a signal for metabolic dysregulation beyond the expected improvements in insulin sensitivity following viral clearance. Therefore, the evidence supports the drug’s continued inclusion as a cornerstone of HCV eradication strategies, provided that access programs are transparently managed.
October 21, 2025 AT 22:53
parth gajjar
They sold us a miracle and left us holding the broken bottle
October 22, 2025 AT 05:50
Maridel Frey
It is encouraging to witness such optimism within the community. Reinforcing early diagnosis and prompt initiation of sofosbuvir‑based therapy aligns with the broader public‑health objective of minimizing hepatic decompensation. As mentors, we should continue to facilitate patient education, ensure adherence monitoring, and advocate for equitable access to antiviral regimens across all socioeconomic strata.
October 22, 2025 AT 12:46