When you pick up a generic pill at the pharmacy, you expect it to work just like the brand-name version. And for the most part, it does. But what happens when new safety risks emerge? Who updates the warning label? And how do you know if your medication is safe? These aren’t hypothetical questions-they’re real concerns for millions of people taking generic drugs every day.
How FDA Safety Alerts Work for Generic Drugs
The U.S. Food and Drug Administration (FDA) tracks drug safety through its MedWatch program, which collects reports of side effects, errors, and adverse events from doctors, pharmacists, and patients. These reports feed into the FDA Adverse Event Reporting System (FAERS), where analysts look for patterns that might signal a hidden danger. For brand-name drugs, manufacturers can update warning labels on their own using something called the Changes-Being-Effected (CBE-0) process. This lets them add new safety information-like a risk of liver damage or a dangerous interaction-without waiting months for FDA approval. It’s a fast-track system designed to protect patients when new evidence pops up. But here’s the problem: generic drug makers can’t do this. Under rules set by the Hatch-Waxman Act of 1984, generic manufacturers must copy the exact label of the original brand-name drug. If a new safety issue arises, the generic company can’t change the warning on its own. They have to wait for the brand-name maker to update their label first, then ask the FDA to approve the change for generics too. That gap means patients on generics might be taking a drug with outdated safety information-even when the brand-name version has a clear warning. And because generics make up over 90% of prescriptions filled in the U.S., that’s not a small issue.Why Generic Labels Are Often Out of Date
Generic drugs aren’t just cheaper versions-they’re legally required to be identical in active ingredient, strength, dosage form, and how they work in the body. But they can differ in things like color, shape, fillers (called excipients), and packaging. These differences don’t affect how well the drug works, but they can affect safety. For example, a generic version of a heart medication might use a different dye or preservative than the brand-name version. In rare cases, those excipients can trigger allergic reactions in sensitive patients. If the brand-name label doesn’t mention this risk, the generic label won’t either-even if the FDA has received dozens of reports about it. The FDA’s Office of Generic Drugs does monitor these issues. Staff review monthly data from FAERS and even do proactive checks when a new generic hits the market. In 2019, they watched the first generic version of Rexulti closely for a full year. No safety signals showed up. But that kind of monitoring is reactive, not preventive. It doesn’t stop problems-it just catches them after they happen. And here’s the kicker: the FDA proposed fixing this in 2013. They suggested letting generic manufacturers use the CBE-0 process too. That would mean if a new safety concern emerged, the generic company could update the label right away, just like the brand-name maker. But nothing has changed since then. As of late 2024, the rule is still under review.
Who’s Fighting for Change-and Why
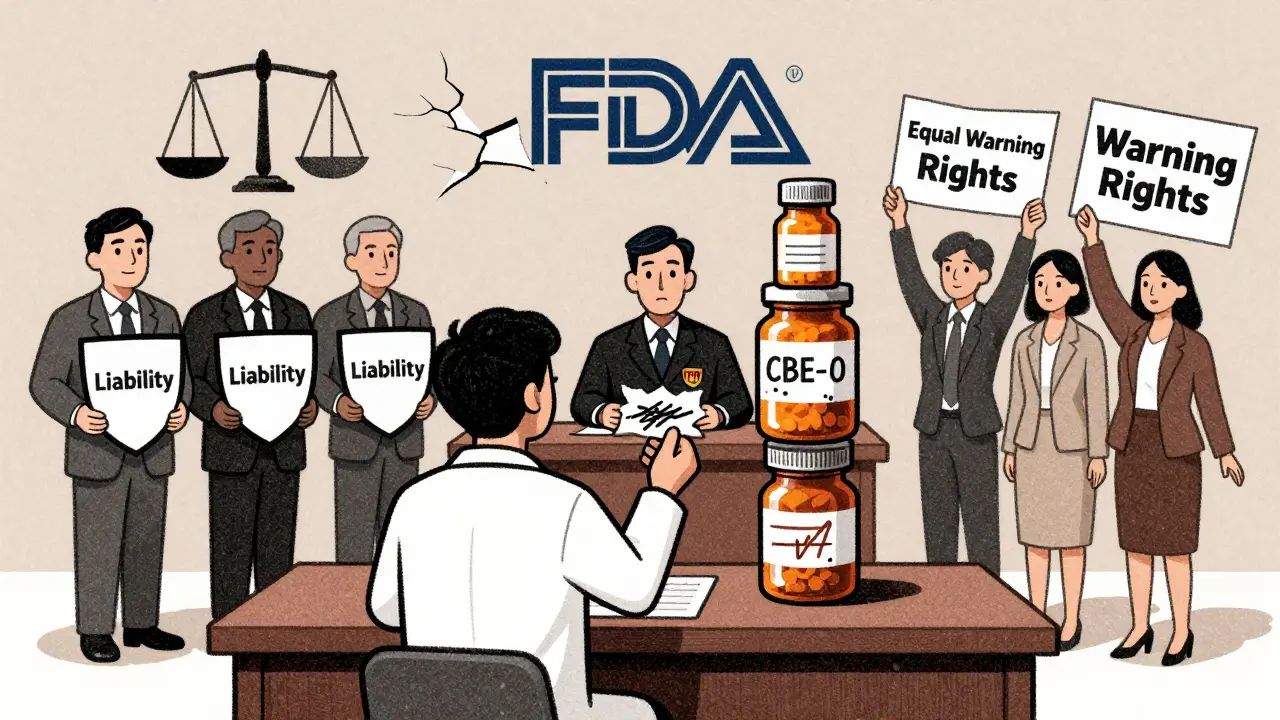
Twenty-seven consumer health groups, including patient advocacy organizations and public interest lawyers, pushed the FDA in 2022 to finalize the rule. Their argument is simple: if insurance companies force you to take a generic, you deserve the same safety information as someone taking the brand-name drug. They point out that patients don’t always know what they’re taking. A pharmacist might switch your medication without telling you. If your label doesn’t warn about a newly discovered risk, you might not realize your symptoms are linked to your drug. On the other side, the Generic Pharmaceutical Association (GPhA) argues that letting generics change labels would open them up to lawsuits. If a generic maker adds a warning, they could be blamed for implying the drug is unsafe-even if the FDA hasn’t confirmed the risk. And if they don’t add it, they could be sued for failing to warn. Brand-name companies have their own worries. If generics can update labels independently, they might be pressured to match every change-even if they disagree with the evidence. That could slow down innovation and raise costs. It’s a legal and ethical tangle. The FDA is caught in the middle, trying to balance patient safety, market competition, and liability risks.What You Should Do If You Take Generic Drugs
You can’t wait for regulators to fix this. Here’s what you can do right now:- Check the FDA’s Drug Safety page regularly. The agency posts alerts for both brand and generic drugs. Look for updates on your medication-even if your label hasn’t changed.
- Ask your pharmacist if your generic drug has the same label as the brand-name version. If it doesn’t, ask why.
- Sign up for MedWatch alerts. You can get email notifications when the FDA issues new safety warnings for any drug you’re taking.
- Report side effects. If you experience something unusual, report it to the FDA through MedWatch. Your report could help trigger a label update.
- Know your excipients. If you have allergies (like to dyes, lactose, or sulfites), ask your pharmacist what’s in your generic. Some generics use different fillers than the brand.
The Bigger Picture: Cost vs. Safety
Generic drugs save the U.S. healthcare system an estimated $300 billion every year. That’s huge. Without them, millions of people couldn’t afford their prescriptions. But safety shouldn’t be the price we pay for savings. The current system treats generics like second-class drugs-same active ingredient, but second-rate warnings. That’s not fair to patients. The FDA has the authority to fix this. They’ve had the proposal ready for over a decade. What’s holding them back? Political pressure. Legal risk. Industry lobbying. But the longer they wait, the more patients are at risk. In 2024, the FDA issued a warning about Oxbryta’s withdrawal due to safety concerns. That drug was brand-name. But what if the same issue had been found in a generic version? Would patients have been warned in time?What’s Next for Generic Drug Safety?
The FDA is paying more attention to complex generics-drugs with tricky delivery systems like patches, inhalers, or extended-release injectables. These are harder to copy exactly, and small differences can affect safety. As more complex generics hit the market, the current labeling system will become even more outdated. The FDA will have no choice but to act-or risk losing public trust. The ball is in their court. Until then, patients need to be their own advocates. Don’t assume your generic drug’s label is up to date. Don’t assume your pharmacist knows the full story. And don’t wait for the system to protect you.Can generic drug manufacturers update their own safety labels?
No, not under current rules. Generic manufacturers must copy the safety label of the brand-name drug and cannot change it without FDA approval. Only brand-name makers can use the CBE-0 process to update labels quickly. This creates delays in warning patients about new safety risks.
How does the FDA monitor safety for generic drugs?
The FDA uses its MedWatch program and the FDA Adverse Event Reporting System (FAERS) to track side effects and adverse events. Staff review monthly reports, conduct proactive surveillance when new generics launch, and investigate patterns linked to specific drugs or manufacturers. They also monitor the Drug Quality Reporting System for contamination or manufacturing issues.
Are generic drugs as safe as brand-name drugs?
Yes, in terms of active ingredients, strength, dosage, and effectiveness. The FDA requires generics to meet the same high standards for quality, purity, and stability. However, differences in excipients (like dyes or preservatives) can affect safety for some patients, and outdated labels may not reflect new risks discovered after approval.
What should I do if I think my generic drug is causing side effects?
Talk to your doctor first. Then report the issue to the FDA through MedWatch, either online or by phone. Your report helps the FDA detect patterns and may lead to a label update. Don’t stop taking your medication without medical advice.
Why hasn’t the FDA changed the rules for generic drug labels yet?
The FDA proposed allowing generic manufacturers to update labels independently in 2013, but the rule hasn’t been finalized. Industry groups oppose the change, citing legal liability risks. The FDA is balancing patient safety, manufacturer concerns, and legal authority under the Federal Food, Drug, and Cosmetic Act. Political and legal pressures have delayed action.


Comments
Lori Anne Franklin
so i just found out my generic blood pressure med doesn’t even have the same warning as the brand? no way. i’ve been taking this for years and never even thought to check. thanks for pointing this out, i’m gonna call my pharmacist tomorrow.
December 26, 2025 AT 12:33
Alex Ragen
Ah, yes-the grand hypocrisy of American pharmaceutical policy: we demand cost-efficiency, yet we refuse to grant generics the same epistemic authority as their branded cousins. The Hatch-Waxman Act, that quaint relic of 1980s legislative compromise, has ossified into a grotesque bureaucracy of patient endangerment. We permit generics to mimic pharmacokinetics-but not pharmacovigilance? How quaintly feudal.
It’s as if we’ve created a two-tiered medical caste system: the wealthy, who can afford the brand, receive timely warnings; the rest, who rely on generics, are left to divine safety from fragmented FAERS reports and pharmacist gossip. The FDA’s inertia isn’t bureaucratic-it’s moral.
And let’s not pretend the GPhA’s liability fears are sincere. They’re simply shield-bearers for a system that profits from obscurity. If a generic manufacturer adds a warning, they’re accused of alarmism; if they don’t, they’re complicit in harm. Either way, the patient loses. The only rational solution? Abolish the label monopoly. Let generics update independently-and let the courts sort out liability. The law must evolve with science, not fossilize in the amber of corporate lobbying.
And yet… here we are. Another decade wasted. Another thousand patients unknowingly dosed with silent risks. The real tragedy? We knew how to fix this in 2013. We just chose not to.
December 27, 2025 AT 00:52
Bryan Woods
This is a really important issue that doesn’t get enough attention. I work in pharmacy and see patients switch between brands and generics all the time without realizing the label differences. It’s not that the drugs are unsafe-it’s that the information isn’t always current. The FDA needs to act, but until then, patients should definitely check the Drug Safety page and ask questions.
December 27, 2025 AT 10:53
Ryan Cheng
Hey everyone-just want to say you’re not alone if this caught you off guard. I used to think generics were just ‘cheap versions’ until I learned how the labeling rules work. It’s wild that a drug can be chemically identical but legally stuck with outdated warnings. If you’re on a generic, take 5 minutes to look up your med on the FDA site. And if you’ve had a weird side effect? Report it. It literally helps save lives down the line. You’re not just a patient-you’re part of the system.
December 28, 2025 AT 02:30
Jeanette Jeffrey
Wow. So we’re letting people die because of corporate greed and lazy regulators? Brilliant. The FDA’s been asleep at the wheel for a decade. And now we’re supposed to be grateful for cheap pills while being kept in the dark? This isn’t healthcare-it’s a scam dressed in white coats. If you’re not outraged, you’re complicit.
December 29, 2025 AT 09:00
Michael Bond
Label updates should be mandatory for both.
December 30, 2025 AT 15:11
david jackson
Let me tell you something that keeps me up at night: I took a generic version of a drug that later got a black box warning… and my label? Still said ‘generally well tolerated.’ I didn’t find out until I saw a news article six months later. Six months. I could’ve been hospitalized. Or worse. And the worst part? The pharmacist didn’t know the label was outdated. They just filled the script. That’s not negligence-it’s systemic failure. The FDA has the data. They’ve had the proposal. They’ve had the warnings. But they’re waiting for a lawsuit before they act? That’s not policy. That’s cowardice. And now I’m terrified to take anything that’s not brand-name-even if I can’t afford it. What kind of world are we living in where your access to safety depends on your bank account?
December 30, 2025 AT 19:11
Shreyash Gupta
lol why are you all so dramatic? generics are fine. if you’re having side effects maybe you’re just weird. also 🤡
January 1, 2026 AT 11:00
Jay Ara
i read this and i felt so bad for people who cant afford brand name. my cousin took generic diabetes med and had bad reaction but doctor said its same as brand so she didnt report. now she knows. please tell more people. its not just about money its about trust.
January 2, 2026 AT 09:18
Jody Kennedy
THIS. IS. HUGE. I just started taking a new generic and I’m going to sign up for MedWatch right now. I’m not waiting for someone else to protect me. If you’re reading this and you take any meds-do the same. It takes 2 minutes. You’re worth it.
January 3, 2026 AT 14:11
Ellie Stretshberry
i never thought about the dye in pills being a problem… i have a rash sometimes and never connected it. gonna ask my pharmacist what’s in mine. thanks for making me think about this
January 4, 2026 AT 06:41
wendy parrales fong
we all just want to feel safe when we take medicine. it doesn’t matter if it’s cheap or expensive-what matters is that we know what we’re taking and what it might do. the system is broken, but we can still look out for each other. share this. talk to your doctor. report your symptoms. small things add up. we’re not powerless here.
January 6, 2026 AT 01:42